Liver cancer is one of the leading malignancies in the world, and 886,000 new cases are diagnosed in 2022 (https://www.wcrf.org/preventing-cancer/cancer-statistics/liver-cancer-statistics/#latest-liver-cancer-data). Hepatocellular carcinoma (HCC) is the major type of primary liver cancer, and the current treatments for advanced HCC include multi-targeted tyrosine kinase inhibitors (TKI), such as Sorafenib or Lenvatinib, and immunotherapy targeting PD-L1 (atezolizumab) or VEGF receptor (bevacizumab). Although significant progress has been made in the diagnosis and treatment of liver cancer, the prognosis for HCC patients is far from satisfactory due to drug resistance and poor responses to immunotherapy. Thus, understanding the underlying mechanisms of drug resistance for HCC has been a long-term challenge in the field.
Cancer cells adopt various adaptive mechanisms to survive harsh conditions in the tumor microenvironment, such as lack of oxygen, acidosis, anti-cancer drugs, and nutrient deprivation. Recent studies discovered that liver cancer cells form a new type of subcellular organelle, termed stress granules, in response to these stresses. In particular, a recent work from Prof. Lianxin Liu’s team at University of Science and Technology of China observed that liquid-liquid phase separation of a protein kinase RIOK1 in HCC cells form stress granules to trap messenger RNA of a tumor suppressor PTEN, which leads to the activation of the pentose phosphate pathways for the cancer cells to survive nutrient deprivation of glutamine or with anti-cancer drug (Donafenib) treatment (Figure 1). They also found an FDA approved small molecular drug Chidamide can be repurposed to target this pathway and overcome drug resistance. This study was recently published in Nature Cancer, one of the premier scientific journals in the cancer field (https://doi.org/10.1038/s43018-024-00886-y).
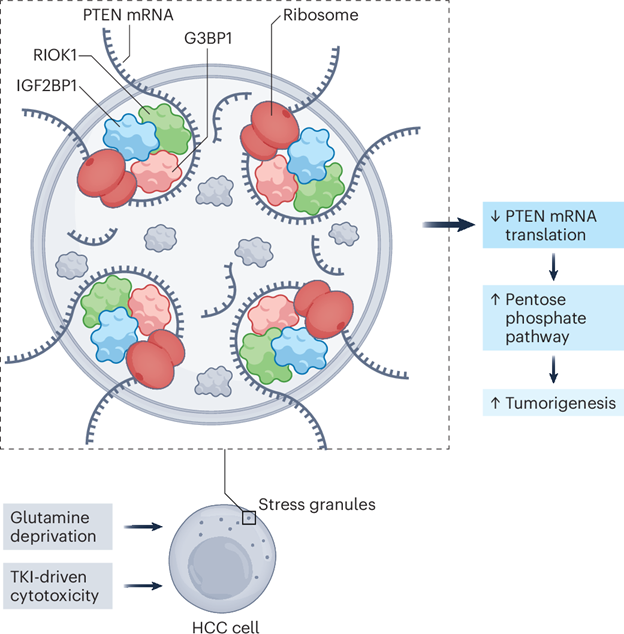
Figure 1. Formation of stress granules for metabolic reprogramming and stress adaptation in cancer
On June 4, 2025, Professor Huiyong YIN and Dr. Shanshan Zhong were invited to give a commentary for this study as a “News & Views” entitled “Stress granules shape metabolic reprogramming and drug resistance” (https://doi.org/10.1038/s43018-024-00886-y). In this commentary, a brief summary of the roles of stress granules in the cancer was described. Trapping a mRNA of a tumor suppressor in stress granule to facilitate metabolic reprogramming represents a novel model of action for cancer cells for stress adaptation. They also pointed out the clinical implication for this study and future directions for this frontier of cancer research.
Professor YIN’s team has been interested in investigating the metabolic reprogramming of liver cancer cells, a core hallmarks of cancer cells that distinguish cancer cells from normal cells. During the transformation of normal cell to cancer cells, cancer cells rewire their metabolic pathways to meet the demands for energy and biosynthesis during rapid cell proliferation. Although the phenomena of metabolic reprogramming of cancer cells, such as the well-known “Warburg effect”, can be dated back one century ago, research interests to understand how cancer cells alter their metabolic pathways have not been intensified until the recent decade.
Our lab has made significant contributions to the field by identifying novel mechanisms by which liver cancer cells take advantage to rewire their metabolic pathways. For example, we observed downregulation of a key enzyme in the glucose metabolic pathways, fructose-1,6-bisphosphate aldolase (ALDOB) during the progression of HCC. Downregulation of ALDOB leads to metabolic reprogramming of glucose in cancer cells favoring cell proliferation through upregulating insulin receptor pathway (Hepatology, 2021), PI3K-Akt (Plos Biology, 2020) and pentose phosphate pathways (Nature Cancer, 2020; Cell Rep Med. 2023). A recent work from YIN lab found that metabolic reprogramming of liver cancer cells can also affect the immune cell (T cell) functions in the tumor microenvironment (Hepatology, 2025).
The research projects in YIN lab are financially supported by grants from National Natural Science Foundation of China, Shenzhen Medical Academy of Research and Translation (SMARF), General research fund from UGC and startup grants from City University of Hong Kong.
Leave a Reply